Our solution is designed to support customer-facing teams with streamlining how they provide and manage their product safety and prescribing information.
With emc compliance, these teams can stay compliant with the current codes when promoting their products to HCPs.
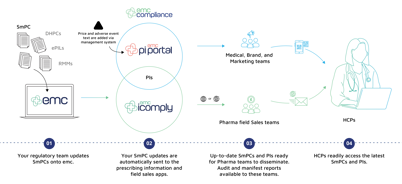
Two applications working together as one integrated solution
The emc compliance solution consists of two applications:
1. The emc pi portal app, which provides you with HTML links/QR Codes to your prescribing information to use within your digital and printed materials
2. The emc icomply app for field sales and MSLs to share medicines information compliantly with HCPs

Real-time updates
Information is kept in sync with the latest SmPC on emc, so that you only need to update information from one source

Audit-ready reporting
Maintain visibility of all your generated links, including when they were generated and by whom, and prepare for your internal and external audits

Maintain compliance
Satisfy the industry requirement to provide a single click to your prescribing information

Generate QR Codes
Simplify your creation and management of QR Codes linked to your prescribing information, with flexibility to meet your presentation needs

Multiplatform capabilities
Designed for multiple ways of sharing information, so you can access your portfolio of products using tablet, mobile or desktop

Highly centralised control
Providing the administrator with an easy-to-use interface to manage portfolios and monitor usage

Online and offline access
Accessing up-to-date SmPCs and Prescribing Information wherever you meet your customers
QR Codes
A simple solution for compliance
In line with 2024's update to the ABPI Code of Practice, in Clause 12.1, emc compliance includes QR Code functionality for linking to the prescribing information.
This means that you have a low-burden, efficient process for managing your prescribing information when adopting QR Codes for your printed materials.
To receive content alerts around this topic, including our newsletter, subscribe below.

How emc compliance supports Pharma
Our integrated portal for automating your compliance
Promotional materials for medicines require the latest Prescribing Information (PI). However, with constant changes to regulated safety information, it is labour-intensive to keep this information up-to-date.
Our emc compliance solution enables Pharma companies to keep digital prescribing information up-to-date while satisfying the industry’s requirement to link to it within a single click, as outlined in the ABPI code of practice (clause 12.4).
This is fully integrated into the emc compliance solution, meaning that when you create a new PI for your medicine, the price and adverse event text will be synced and added to the app which your MSLs use.

Our compliance application for customer-facing teams
To meet regulatory requirements of the MHRA Blue Guide and ABPI Code of Practice (as well as Pharma codes of practice), customer-facing teams are required to have access to the most up-to-date version of the SmPC for each product they promote when meeting HCPs.
MSLs now also follow a working-from-home or hybrid-working model, meaning that screen sharing with HCPs has become a common part of everyday work life.
With emc compliance, these teams can present their products in whichever setting they prefer, online or offline, in an online call or physically face-to-face. Linked to compliant information on emc, the MSL can use an app to ensure that information on your portfolio of products is to hand.
It also includes a full reporting and management system that allows your teams to demonstrate compliance.
Get in touch to learn more
What our customers say
The two main reasons we use both emc compliance features is to help us maintain high standards when it comes to supporting our teams with remaining compliant when they are in the field, as well as all digital assets linked to the latest prescribing information automatically. Second, to support our monitoring efforts and maintain transparency when it comes to auditing how well we are adhering to our own high standards."
- Digital Strategy Lead, Gilead
CASE STUDY
Automating compliance for Pharma sales & marketing teams
Learn how to ensure Pharma regulatory compliance for MSLs and marketing staff with Datapharm’s integrated software solution.

Frequently Asked Questions
While emc is a great resource that hosts product and safety information for more than 9,000 medicines, both that and other websites (corporate or microsites) do not work offline.
When meeting customers, your sales reps will be able to access SmPCs both online and offline by simply accessing the app. Every action on the app is logged in the management system so your Compliance team is able to provide reports for auditing purposes.
The emc icomply and emc pi portal platforms are now part of one complete solution, the emc compliance solution, supporting Pharma commercial and regulatory teams with their compliance challenges.
Some companies keep all their regulatory documents stored in their shared cloud. However, this works only when the user's device is connected to the internet. If a new document is not promptly added into the shared folder, the risk is to provide the customers with an outdated SmPC.
emc compliance gives the system administrator full control over which user can have access to each document, providing a complete overview of who viewed the available SmPCs and identifying potential non-compliance.
The compliance app for MSLs has a very intuitive and user-friendly interface. Your customer-facing team will be just required to download the app, available for free on both the Apple and Google Play stores, and they are ready to go. New documents and updates will be automatically available on the reps’ devices.
Through the management system, you will have complete flexibility to remotely manage your sales team and their SmPC portfolios.
emc pi portal is the back end platform that allows emc compliance solution users to publish their prescribing information (PI) providing quick, one-click access and maintaining compliance.
Due to the integration functionality within emc compliance, when you create a new Prescribing Information for your medicine, the price and the adverse event text will also be added to your products within the customer facing app.
Your customer-facing teams will have access to up-to-date Prescribing Information that can be available both online and offline.
Explore the latest product updates and news

Whitepaper
HCPs prefer digitally-delivered content
A whitepaper revealing UK healthcare professionals' views on medicines documentation

Blog
QR Codes: Best practices for Pharma
With the anticipated update to the ABPI Code expected to include the use of QR codes for linking to prescribing information from printed materials, it's a good time to consider some key areas for adopting this technology.
Want to learn more about our emc compliance solution?
Chat with us
Phone: +44 1372 371444
Email: [email protected]
Address:
Cassini Court, Randalls Way, Leatherhead, Surrey, KT22 7TW, United Kingdom