Blog
Submit once, publish everywhere: How emc helps Pharma reach the UK healthcare ecosystem
Jul 21, 2025
emc (the electronic medicines compendium) is more than a medicines information website, it’s the database that distributes Pharma’s medicine information into all major channels within the UK healthcare ecosystem.
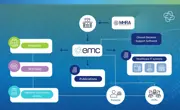
It’s vital to ensure that your product information is put in front of your audience where they work and whenever they need it. emc itself receives tens of millions of visits annually. But beyond emc, did you know that medicines information published on the emc database reaches a large healthcare ecosystem, including clinical decision support systems and established medicines information publications?
The trends: How HCPs and patients prefer to receive information
Building a platform to reach your audience online takes a huge amount of time and investment, developing partnerships and enabling the tech to run smoothly. But importantly, if you’re a supplier of medicines, a medicines information resource needs to be a place which HCPs trust, and regularly go back to for the medicine information which is so vital to their work (in the EPG Health Report 2023, 74% of HCPs considered independent medical websites as either very important or critical).
For patients, their information journey often contrasts with HCPs (for example, a large proportion of patients use mobile to access medicines information on resources like emc, whereas HCPs still tend to use desktop, at least for now), but there is a clear indication that trustworthy, independent resources are also valued by patients.
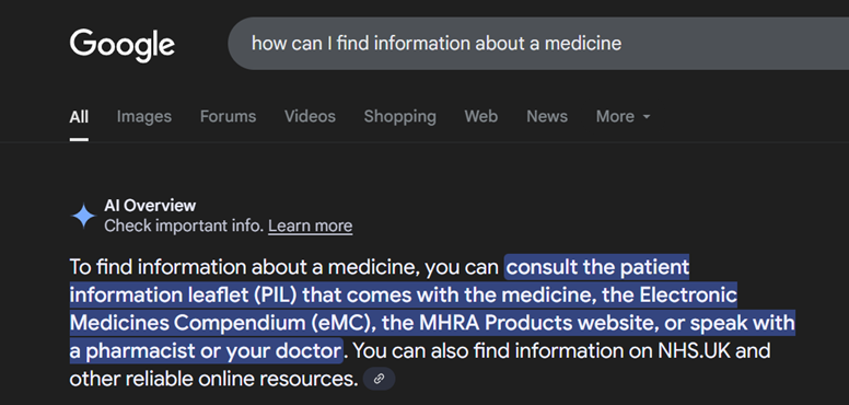
Arguably the biggest recent shift in online search sees users finding AI-generated results from tools such as Google’s AI search or ChatGPT. But are patients really trusting in these tools to return accurate and trustworthy information? When we asked patients how they prefer to find medicines information (emc user survey, 2025), just 4% said they use an AI tool, while 28% said they used patient-orientated websites.
Interestingly, AI responses seem to be very capable of finding resources which are trusted and used frequently by healthcare professionals and patients (though perhaps less so for providing accurate clinical or directional information).
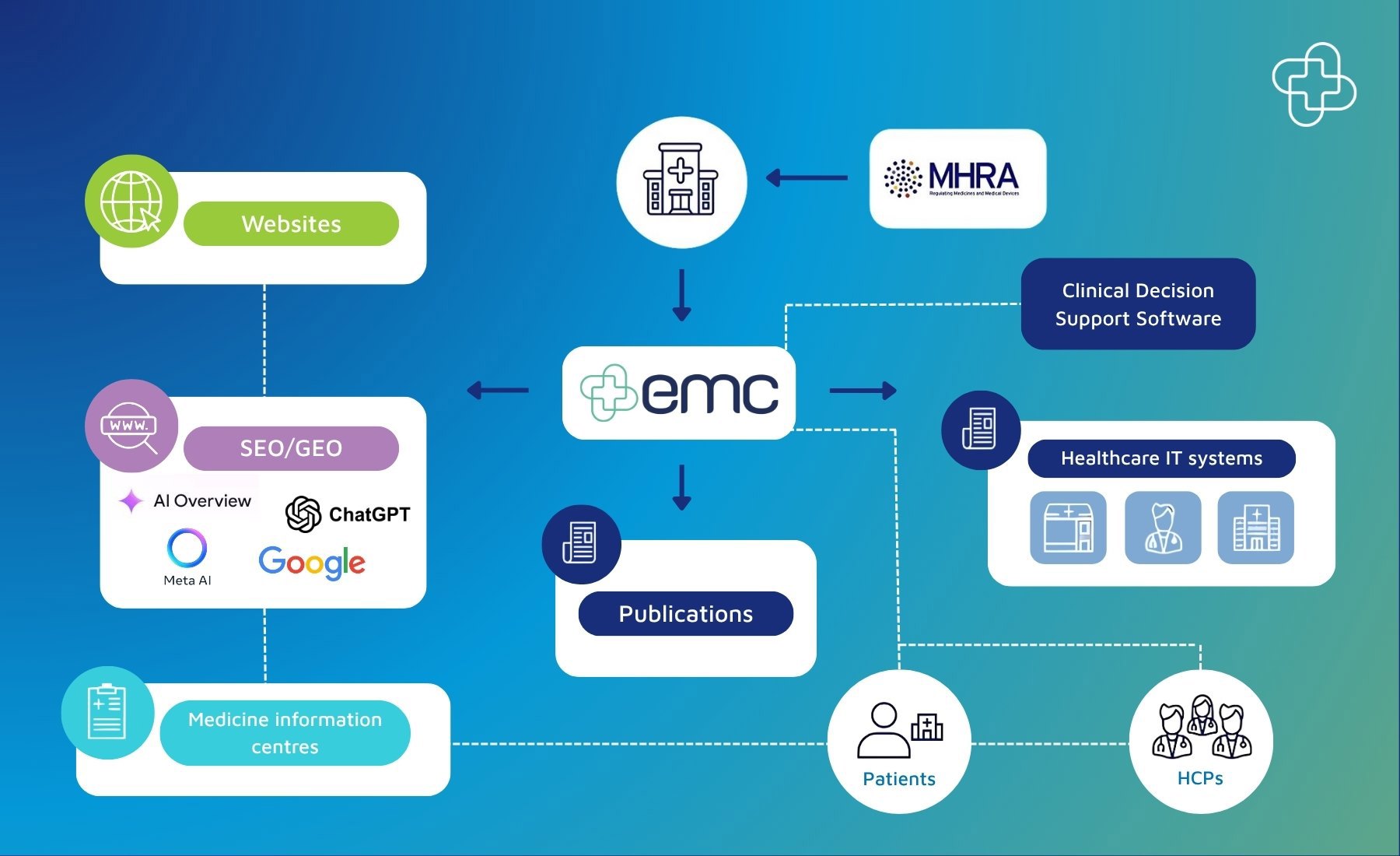
How medicine safety information is distributed from emc to the wider healthcare ecosystem
emc, as a database of information, is used by virtually all medicines information providers in the UK. This includes websites, healthcare IT systems and publications - and through Datapharm’s partnerships, covers every HCP and prescription (estimated at 1.21 billion prescriptions) in the UK.
Once you publish your approved content, such as SmPCs and PILs, on emc, this is fed through to health information websites used by HCPs and patients, medicines information publications, and integrated into all the major third-party providers, including GP, hospital and pharmacy IT systems.
The integration with these healthcare system providers (such as clinical decision support software in the primary care setting), means it is easier for trusted medicines information to be accessed at the point of consultation, prescription and dispensing, and ultimately ensures the HCP has the latest information to hand.
What does the future hold for the dissemination of medicines information?
With the new data standard, FHIR, being adopted by the NHS, and throughout Europe, Pharma’s medicines information can reach beyond the UK healthcare ecosystem, both in terms of audience size and application. Thanks to FHIR, a standard structure for this information opens up a world of possibilities - for example, the information that Pharma publishes could be:
- Linked to the patient health record
- Translated accurately into other languages;
- and even be used to drive efficiencies through interoperability with other healthcare systems (such as immunisation and vaccination systems).
Another key reason data standards are so important is the subject on everyone’s lips, Artificial Intelligence (AI). While healthcare professionals and patients aren’t overwhelmingly using AI generated responses to find answers to their questions about medicines (yet), these tools will only see more adoption, not less, in the future, and therefore present a significant safety risk if not embraced correctly.
Using AI itself, is not the main challenge Pharma face, more so it’s the data behind it. We should always be questioning where the AI sources its information from (you’ll find this an interesting exercise), and be mindful that these tools can hallucinate, ending up in very inaccurate, or nonsensical, answers on occasion.
Therefore, where AI is used, it should go hand in hand with trusted, regulated and fully structured medicines information. It is because emc’s medicines information is structured to be optimised to engage with AI tools, it cannot be taken out of context, and it follows industry standard, e.g. if it’s a type of allergy, it’s accepted by the industry as one, and it’s defined within the scope of a product information standard.
emc, with its product information structured in FHIR, provides a solid foundation for Pharma to work hand in hand with AI tools in the future. Datapharm is excited to be looking at where AI fits in within this new era of digital medicines information, as it continues to ensure Pharma's up-to-date product information is distributed to the vast array of stakeholders throughout the UK healthcare ecosystem.
Learn more about emc
emc has been used for decades as a trusted source of medicine safety information for HCPs and patients. Once a printed publication, it became digital in 1999 and has continued to evolve and serve the healthcare industry with up-to-date, compliant, non-promotional materials on medicines.
If you would like to learn more about how emc is supporting Pharma with distributing its medicine product information, get in touch with our team.