Why MILE chose to partner with Datapharm
Making information accessible through a trusted source
Core to MILE’s mission is providing accessible, accurate and balanced medical information to support everyday healthcare decisions. It was therefore essential to make this information available via channels HCPs use and trust. emc, trusted by millions of UK HCPs and patients annually as their vital source of medicine safety information, became a natural choice of channel.
Many HCPs value having an independent one-stop shop for their medicines information. During Datapharm’s annual emc user survey, 97% of UK HCPs said they trust emc for their medicines information, with 81% stating emc is their first choice. Moreover, 74% of HCPs globally consider independent medical websites as either critical or very important for accessing scientific content.
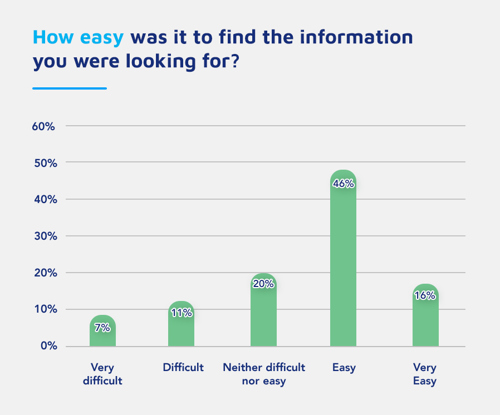
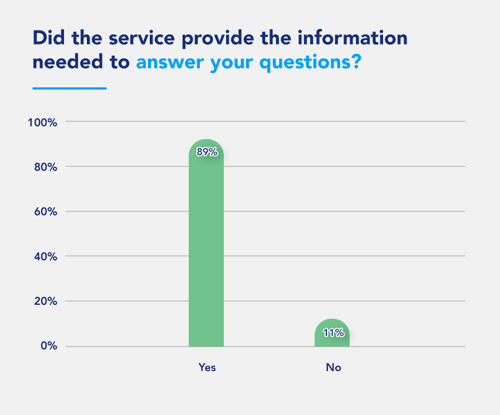
The SRD service on emc enabled HCPs to find answers to their questions 24/7 on a platform they frequently use and trust today, meaning Pharma could reach those growing number of HCPs who search and expect to find online answers. In turn, the HCPs can easily find and access the information to help make the best treatment decisions for their patients.
For Pharma, the service provides an additional self-serve channel with valuable insights on the searches that HCPs are making about their medicines online, increasing their knowledge and ability to ensure they are providing answers to the right questions.