Case Study
Examples of how emc is assisting the NHS in improving patient safety
Aug 15, 2024
A first-hand look at how Pharma’s medicines information is being effectively communicated to key medicines information stakeholders within the healthcare ecosystem.

We are now accustomed to medicines information constantly changing in the Pharma industry. This happens for a number of reasons - for example, due to the addition of new medicines, new medical studies or specific safety reasons. As so many healthcare services rely on up-to-date medicines information, the effective communication and synchronisation of these changes is crucial for patient outcomes.
emc, providing digital medicines information to healthcare since 1999, is a pivotal part of the healthcare ecosystem. It holds a vast amount of product information which captures medicines information changes throughout time. When utilised with the right technical expertise, emc can be a critical source for maintaining digital healthcare information resources and tools.
Supplying up-to-date medicines information to UK healthcare services
Reliable, timely, accessible, and accurate Medicines information is crucial for several key online services used across the NHS, including:
- NICE’s Medicines Awareness Service
- The Refrigerated medicines stability tool, provided through the SPS
- NHS Medusa Injectable Medicines Guide
- NHS BSA’s dm+d database
The NHS Specialist Pharmacy Service needs to understand changes in product information when producing the NICE Medicines Awareness Service and the SPS Refrigerated Medicines Stability Tool which hosts information on over 500 refrigerated medicines. The Medicines Awareness Service is a daily or weekly email with links to current awareness and evidence-based information on medicines and prescribing.
The information used in the Refrigerated Medicines Stability Tool includes maximum time and temperature range for storing a medicine outside of its recommended range (excursion) and whether the medicine can be returned to the fridge, proving a valuable source of information for both HCPs and patients.
NHS Medusa is a resource made available to trained NHS healthcare professionals (HCPs), providing guidance on the preparation and administration of injectable medicines. HCPs using the service need to be kept informed of changes in product information for creating and maintaining the content within the Medusa Injectable Medicines Guide.
The NHS Business Service Authority (BSA) oversees the dictionary of medicines and devices (dm+d) which is vital to achieving interoperability of medicines information across different IT systems used between a variety of healthcare settings.
A key part of maintaining the dm+d database content is understanding changes in product information. Having regularly updated information enables healthcare professionals to be confident that they have the latest information in front of them and ultimately helps avoid prescribing and dispensing errors.
How Pharma’s medicines information is distributed via Datapharm’s emc Summary of Product Characteristics (SmPC) change reports
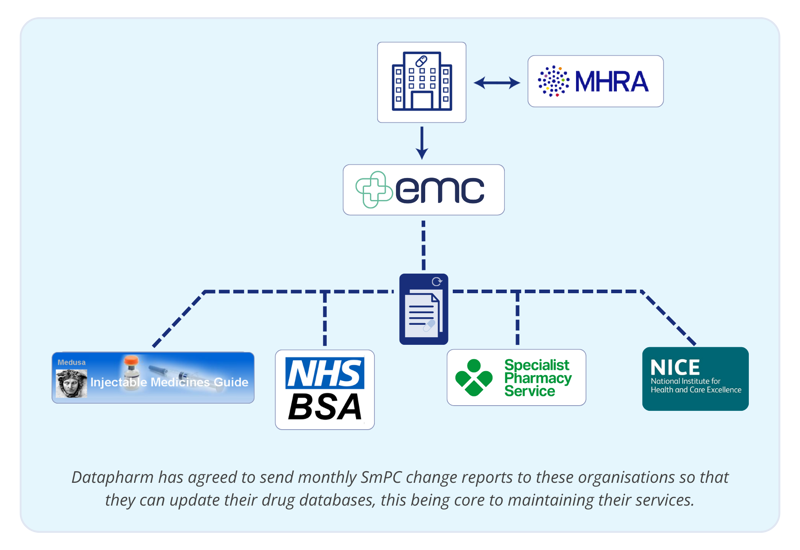
NHS services receive emc SmPC change report for easy access to the most up-to-date information about medicines when providing patient care.
Making all this happen not only required the technical knowledge of Datapharm’s development team, but also establishing important relationships with these healthcare organisations. emc, trusted by over 97% of UK HCPs frequently looking for medicines information, was the natural source of choice while being backed by fully dedicated support and technical teams to ensure the continuous and effective distribution of information.
Learn more about maximising the impact of medicines information
Datapharm continues to collaborate with a wide range of vital healthcare industry stakeholders, supporting better patient outcomes on behalf of the Life Science industry. To learn more about how Datapharm uses medicines information published on emc, get in touch with our team.
About these organisations
The Specialist Pharmacy Service (SPS) is a valuable resource for pharmacists and other professionals involved in all aspects of buying, making, and using medicines. Funded by NHS England and Improvement, SPS brings together experts to provide impartial advice on topics such as assessing liver function, choosing appropriate medications, and understanding patient group directions. Their main purpose is to improve the use of medicines, helping people live longer, fuller lives.
The National Institute for Health and Care Excellence (NICE) is an independent organisation established by the UK Government in 1999. Its mission is to address variations in healthcare availability and quality within the NHS. NICE provides national guidance and advice to improve health and social care, ensuring efficient patient care while maintaining value for taxpayers.
NHS Medusa is a web-based resource for guidance on injectable medicines. It was developed by the injectable medicines guide (IMG) multidisciplinary advisory group, led by Imperial College Healthcare Trust. It is hosted by the NHS Wales Informatics Service and can be accessed via the internet or downloaded and made available through an organisation’s intranet.
The NHS Business Services Authority (NHS BSA) is an Arm’s Length body of the Department of Health and Social Care (DHSC). It manages around £48 billion of NHS spend annually, providing critical central services to support the priorities of the NHS, government, and local health economies.